 The team led by Dr. David Ho of Rockefeller University and Dr. Chi-Huey Wong of the Genomics Research Center of Academia Sinica in Taiwan has reported a DNA-based vaccine candidate for avian flu which shows promise in mice in the newly published Proceedings of the National Academy of Sciences of the United States of America (PNAS) Journal. This work has paved a way for developing effective preventive medicine to avoid the globally dreaded possible pandemic.
The team led by Dr. David Ho of Rockefeller University and Dr. Chi-Huey Wong of the Genomics Research Center of Academia Sinica in Taiwan has reported a DNA-based vaccine candidate for avian flu which shows promise in mice in the newly published Proceedings of the National Academy of Sciences of the United States of America (PNAS) Journal. This work has paved a way for developing effective preventive medicine to avoid the globally dreaded possible pandemic.
It is known that a type of glycoprotein molecule called Hemagglutinin (HA) found on the surface of the H5N1 virus is used by the virus to infect host cells in the first step of its attack. The team, in collaboration with Dr. Alice Yu and Dr. Ting-Jen Cheng at the Genomics Research Center, analyzed HA sequences from various H5N1 strains. As a result, they designed an artificial HA gene that represents a common sequence of HA from all reported H5N1 strains and named it “Consensus HA”. The “Consensus HA” DNA sequence was then inserted into a circular piece of DNA for replication in E. coli and became the H5N1 DNA vaccine.
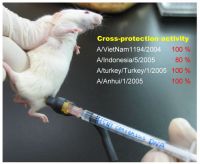
Then, mice immunized with the H5N1 DNA vaccine were exposed to different types of the avian flu virus, including the Vietnam strain, Indonesia strain, Turkey strain, and Anhui strain, since these viruses represent most current avian flu cases throughout the world.
The result was very promising; it showed that 80% of the mice survive from the high-dose challenge by the Indonesia type virus and 100% of the mice survive from the challenge by any one of the other three types, while all the mice without immunization die after the challenge. Antibodies as well as cellular immune responses have been detected in these mice.
It is worth mentioning that several known DNA-based vaccines have shown promise but failed to elicit a strong immune response in vivo. To solve that problem, this team has used an electroporation device developed by ICHOR in San Diego to inject the vaccine into the muscle, which generated a brief electrical pulse to help the DNA enter the cells. Delivered this way, the DNA-based vaccine induced quite drastic immune responses, and protected mice against most of the avian flu strains.
While flu viruses will continue to mutate rapidly, this vaccine strategy could provide a fast, affordable, and easily modifiable approach to combat avian flu, the researchers suggest.
Currently, this DNA vaccine is being produced by the Development Center for Biotechnology (DCB), a local non-profit biotech organization. DCB is also conducting the animal safety test which is projected to be completed by the end of 2008. This vaccine will be submitted to the Department of Health and the FDA for approval as investigational new drug (IND) so that a phase-1 clinical trial in humans can be carried out in the future, if necessary.
This research was sponsored by the Centers for Disease Control (CDC) in Taiwan since 2006.”We are so glad that the researchers from Academia Sinica are willing to devote their time and efforts to help resolve this important public health issue. Only in three years, they have come to such an incredible achievement”, said Dr. Ding-Ping Liu, the director of CDC Vaccine Center.The PNAS article: “A consensus-hemagglutinin-based DNA vaccine that protects mice against divergent H5N1 influenza viruses” has been published on the web site, and is also highlighted in the Summary Report of the Journal.


