Liver cancer, commonly referred to as HCC (the abbreviation of Hepatocellular carci-noma) scientifically, has another nickname – Silent Killer. It is Silent because the majority of patients can feel nothing, it is a “Killer” because oftentimes it is already fatal when detected.
MRI is being used currently to detect any irregular tissues inside the liver. In able to clearly display the differences between regular cells and cancerous cells, the technicians would give the patient orally or through injection a certain kind of agent to yield better display. Liver stores most of the iron inside our body; therefore, iron-related nanoparticles have been used as agents for this matter. In the MRI aspect, iron prone agents are cate-gorized as T2-contrast. One of the hot pursuit topics in Nano-Science is to develop better nano-particles as the agent to yield better display and minimize side effects.
Through the collaborative work, Dr. Michael Hsiao of GRC and Dr. Ru-Shi Liu of NTU, Dr. Da-Hua Wei of KMU has successfully developed a new nano-material named as FePt@Kao. They proved it not only effective in detecting early onset liver cancer in the mouse model but also has the potential in treatment utilizing magnetic fluid hyperthermia (MFH) methods and can be used along with chemotherapeutic drugs. Their study is published in the January 28, 2020 issue of Chemistry of Materials with a cover page coverage.
Dr. Ming-Hsien Chan, first author of the article explains that the commercially available agents used for MRI currently may cause side effects like nausea, allergic reactions, or even kidney injuries. The nanoparticle which they named FePt@Kao, is an iron and platinum compound locked within kaolinite layers. Professor Da-Hua Wei said “kaolinite is a clay mineral used for ceramics. While FePt nanoparticles proved better results for T2-contrast MRI, kaolinite turned out as an excellent carrier to deliver FePt inside lab mice to arrive at the desired destination within their livers”. Due to the fast-growing tendency of cancer cells, more blood veins to supply nutrients to cancer cells is an ac-companying necessity for the tumor, therefore, more of the FePt@Kao agent will reach the cancerous region to give better visibility to MRI.
In the sandwich-like structure of FePt@Kao, kaolinite is chemically stable and provides a uniformly distributed pore structure with high absorption capability, therefore it has rooms for additional substances such as chemotherapeutic drugs.
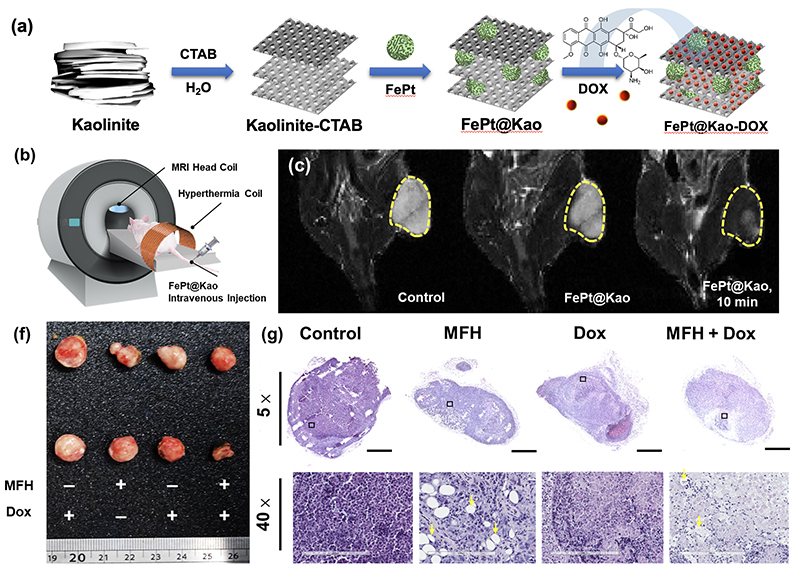 |
| (a) Preparation of FePt@Kao-DOX, as shown in a schematic diagram of the chemical method. (b) Simulated imaging showing the I.V. drug administration (c) In vivo MRI T2-weighted imaging of mice after injection with 100 μL of FePt@Kao in PBS (concentration of 10 mg/mL). (d) collected tumor tissue and (g) H&E staining. The full tumor image was obtained with a visual magnification of 5× , and the 40× images of the tumor. |
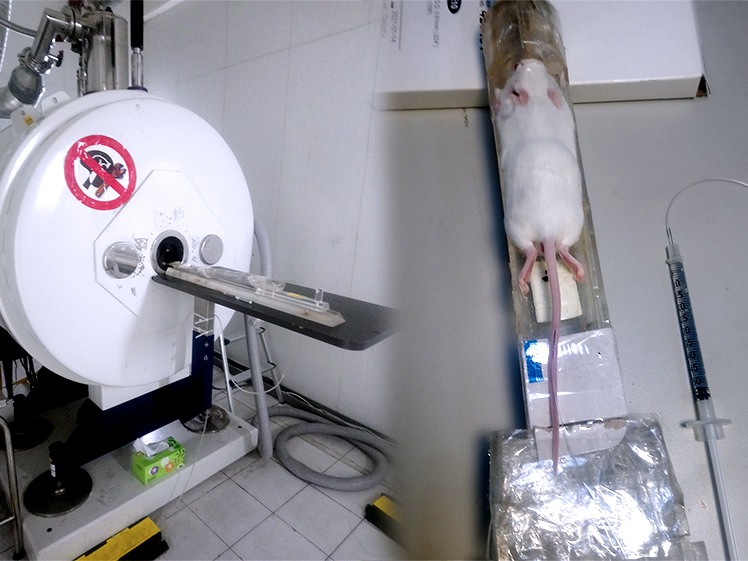
As for adopting the MFH method, it shows a whopping leapfrog effect. In their study, a vibrating sample magnetometer shows that the magnetic field of the FePt@Kao nano-particles is 78% higher than that of the regular FePt nanoparticles. For 20 mg of FePt@Kao under magnetic force, it has an increased heating capacity as to reach 42.5 ℃ in 90 seconds, and it is known the cancer cells will not survive at 41 ℃. A test with locally treated magnetic force on mice showed that magnetic fluid hyperthermia can remove the cancer cells completely. Furthermore, if kaolinite is loaded with chemotherapeutic drugs such as doxorubicin (Dox), it can reside within the liver to inhibit further tumor cell growth. Professor Ru-Shi Liu pointed out that in combination with MFH and chemo-therapeutic drugs, this platform provides a cocktail-type treatment ability, which has made great contributions to human medical progress.
 |
The team has aimed to develop nanomaterials to provide multiple functions for a variety of illnesses. They believe by adjusting the amount of kaolinite, it is possible to develop different nanoparticles for different purposes. Since this material can improve imaging display, be used for magnetic fluid hyperthermia, as well as carrying drugs to the targeted destination within the body, the team is excited and optimistic.
The research paper can be read online at: https://pubs.acs.org/doi/10.1021/acs.chemmater.9b03552