A team led by Dr. Li-Jung Juan of Genomics Research Center, Academia Sinica, reported an unexpected function for Naa10p, which is primarily known to acetylate nascent peptides from ribosomes, in repressing energy expenditure, thermogenesis and beige adipocyte differentiation. These results, published online in the journal Molecular Cell on Aug 12, also suggest inhibiting Naa10p enzymatic activity can be a promising strategy in treating obesity.
Diet-induced obesity (DIO) is a serious health, society and economy issue worldwide. Obesity greatly increases the risk of many life-threatening diseases, such as type 2 diabetes, cardiovascular diseases, stroke, and cancers. DIO is resulted from the pathological expan-sion of lipid-storage white adipose tissue (WAT) caused by the enlargement of adipocyte size (hypertrophy) and/or the increase in adipocyte numbers (hyperplasia) due to excessive food (energy) intake and/or defective energy consumption including deficiency in brown adipose tissues (BAT)/brown-like beige adipocytes. Beige adipocytes that burn fat are a specific subset of brown-like cells found in subcutaneous WAT. Mice deficient of beige adipocytes develop systemic metabolic dysfunctions and obesity. Since beige adipocytes can enhance energy consumption, they have emerged as a new target for treating obesity. However, how beige adipocytes are form remains largely unknown.
Dr. Li-Jung Juan Lab has been studying the mammalian N-α-acetyltransferase 10 protein (Naa10p) which co-translationally acetylates the N-terminal α-amine group of approxi-mately 38% of human proteins. Her Lab reported in Journal of Clinical Investigation that Naa10p is oncogenic by facilitating DNA methyltransferase 1-mediated tumor suppressor gene silencing (https://www.jci.org/articles/view/42275). Moreover, Naa10p has a critical function in early development. Mutations in human Naa10p have been associated with severe developmental defects including infancy death in Ogden syndrome, of which pa-tients rarely survive after one and half years old in age. Consistently, Juan Lab previously reported in Molecular Cell that Naa10p is required to maintain global DNA methylation and genomic imprinting in mouse embryos and embryonic stem cells (http://www.genomics.sinica.edu.tw/2015/index.php/en/news/news-archives/524-role-of-n-acetyltransferase-10-protein-in-dna-methylation-and-genomic-imprinting). The observation that Ogden pa-tients lack subcutaneous fats suggests that Naa10p might regulate metabolism and energy homeostasis.
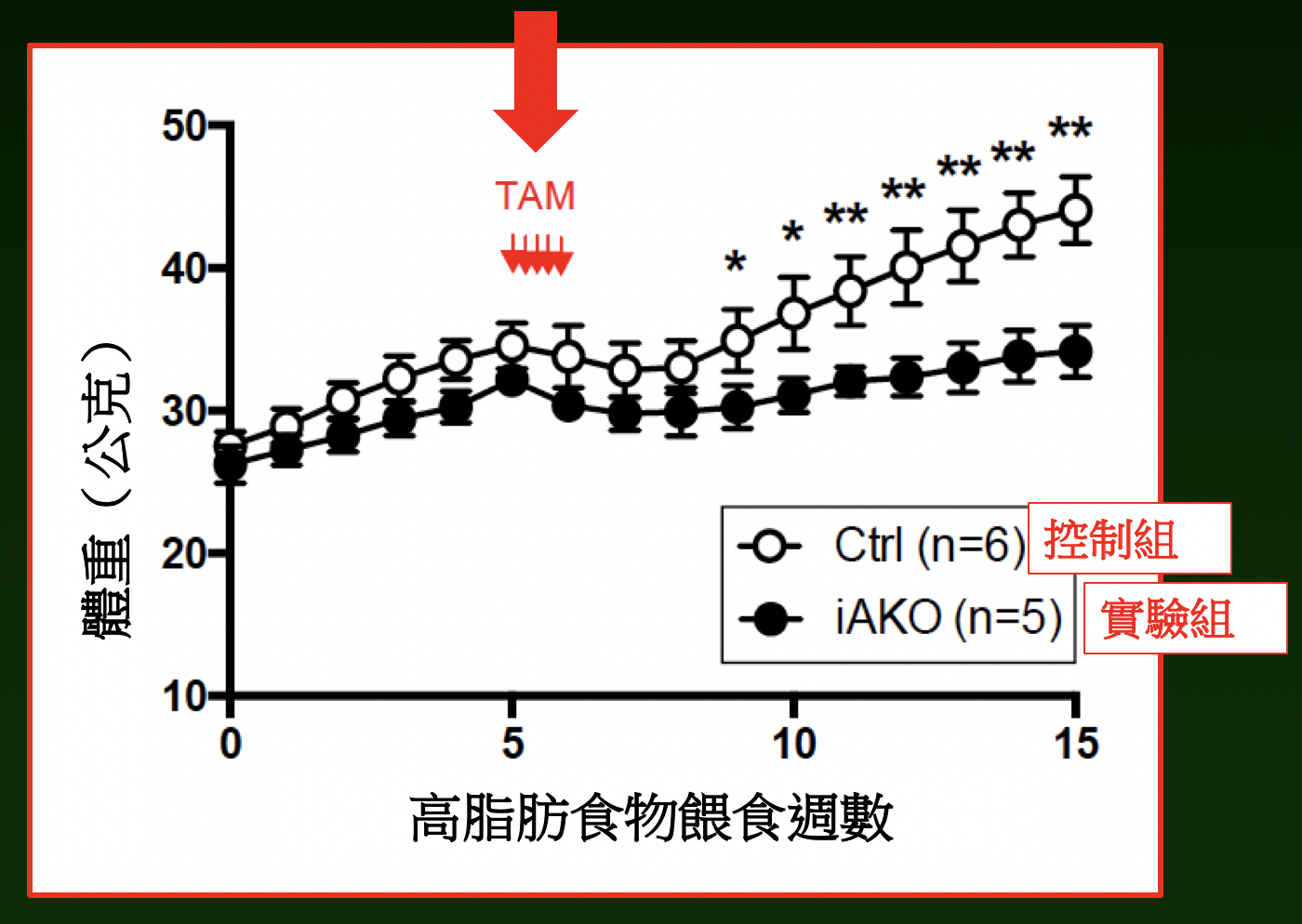
|
| Adipose-specific Naa10p knockout mice maintained same weight while the control group showed a 30% increase in weight with high-fat diet at week 15. |
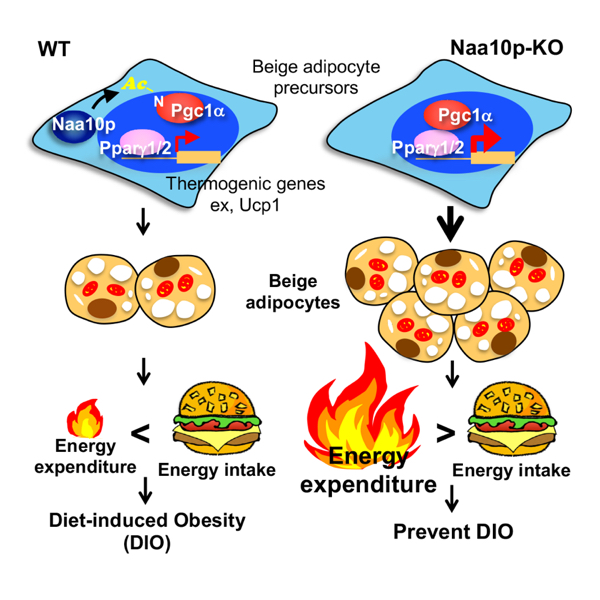
In their latest study, it reports that knocking out (KO) the Naa10p gene from mice prevents high fat diet-induced obesity, promotes formation of beige fats, and increases energy ex-penditure and thermogenesis. Adipose-specific Naa10p KO further confirmed an adi-pose-specific role of Naa10p in preventing beige adipocyte biogenesis. Mechanistically, Naa10p-mediated Pgc1α N-terminal acetylation inhibits thermogenic gene expression likely through compromising the interaction between Pgc1α and Ppaγ. Moreover, Naa10p mRNA level positively correlates with obesity in mice and humans. This study thus pro-vides strong evidence linking obesity to protein N-terminal acetylation. Inhibiting Naa10p enzymatic activity could be a promising treatment for controlling diet-induced obesity.
Together it is clear that Naa10p is required for early development; however, as one grow, excessive Naa10p can turn from a good guy to a bad guy, and may cause obesity and even promote cancers. In their experiments, the adipose-specific Naa10p KO in adult mice not only shows no signs of developmental defects, but also has no symptoms of diet-induced obesity. Inhibiting Naa10p enzymatic activity in adult adipose tissues might just be a new cure to the obesity problem!
The study was primarily corresponded by Dr. Li-Jung Juan. Most experiments were carried out by the first and co-corresponding author Dr. Chen-Cheng Lee, an Academia Sinica Postdoctoral Scholar at Juan Lab, with critical help from Yi-Chun Shih, Ming-Lun Kang and Ramanan Devaraj from the same lab. Collaborators include Dr. Yi-Cheng Chang and Lee-Ming Chuang from Department of Internal Medicine, National Taiwan University Hospital.

|
| Left to right: Ramanan Devaraj, Chen-Cheng Lee, Li-Jung Juan and Ming-Lun Kang |
The research article can be read online at: https://www.sciencedirect.com/science/article/pii/S1097276519305647?via%3Dihub
News and Views at Nat Struc Mol Biol: https://www.nature.com/articles/s41594-019-0310-2
Academia Sinica News: https://www.sinica.edu.tw/en/news/6353

