As the heat rises in the summer, so does the threat of Dengue fever outbreak. Despite of intensive research, there is no effective pharmaceutical treatments for Dengue fever. Personal protection measure to avoid mosquito bites and environmental management to prevent mosquito breeding remain the only available strategies to prevent transmission. In the latest Nature Communications, Dr. Edmond Hsieh’s research team reported their newest findings in treating Dengue fever. This study not only provided a possible therapeutic strategy to treat Dengue fever, but also opened a new avenue for exosomes research field.
Back in 2008, Hsieh’s team was the first to identify the key reason explaining why Dengue fever is so dreadful. They found that Dengue viral infection causes extensive hemorrhage and triggers immunemediated cytokine storm. The patient's immune system becomes out of control and causes treatment difficulties.
At the time, they found that CLEC5A, a C-Type lectin receptor on macrophages, is a key factor for Dengue infection. They further generated specific antibody to against CLEC5A and successfully increased the survival rate of Dengue infected mice from zero to 50%.
However, no matter how hard they modified the antibody, the effectiveness of the treatment still could not break the 50% mark.
"There must be other key pathways yet to be identified!" Dr. Hsieh decided to investigate further. The first author of this paper, Song Pei-Shan, a Ph.D. student at YangMing University, has devoted many years and finally found that dengue virus does not use a simple pathway to cause such deleterious health effects! The point of shock that causes cytokine storms is not only acts through CLEC5A on macrophages, but also via activating platelets.
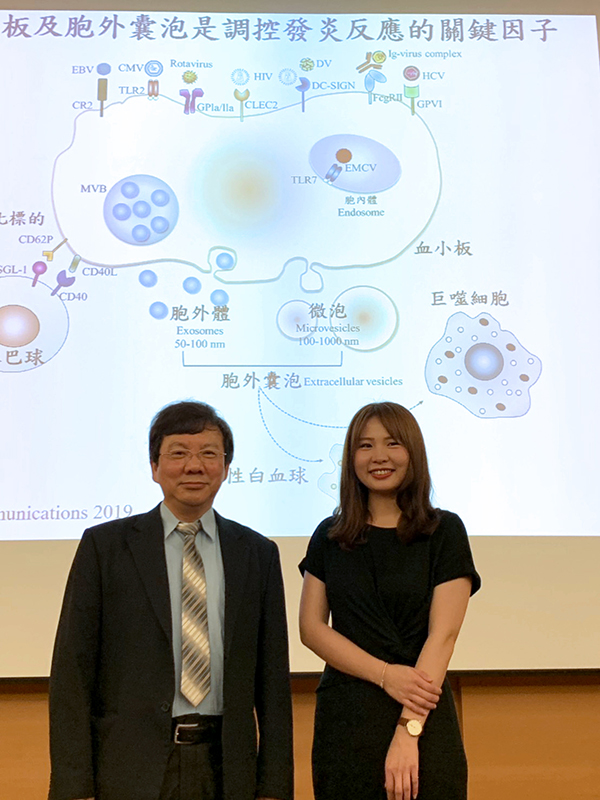
|
| Team members(from left to right): Dr. Edmond Hsieh and first author Ms. Pei-Shan Song, Ph.D. student |
There are many types of cells in our blood. Besides white blood cells including neutrophils and macrophages, there are also red blood cells and platelets. Platelets are known to help blood clogging and slow down bleeding for wound healing.
In this study, the team discovered that dengue virus can activate platelets to induce release of tiny substances, called “extracellular vesicles (EVs)”, or, exosomes. Because these vesicles are so tiny, traditionally they are considered insignificant, or, garbage dumped by cells.
Only lately, exosomes have been taken more seriously. Every kind of cells can release their own kind of exosomes, and these tiny nanoscale pouches containing cellspecific RNA and proteins can affect the physiological functions of other cells. To be precise, the EVs are grouped into two categories: microvesicles are the ones with diameter greater than 100 nm, and exosomes are the ones with diameter less than 100 nm. It was unclear if these EVs have any roles in infectious diseases, and the Hsieh’s team wanted to find out.
It turned out that exosomes secreted from platelets after dengue viral intrusion have morphed into target oriented weapons, much like a prepositioned cruise missiles. These vesicles would turn around to attack their allies in the white blood cells. The attack is fierce and precisely targeted toward CLEC5A and TLR2 on both neutrophils and macrophages.
As a result, standard procedures of the immune system kicked in. Besides the cytokine storms stirred up by the macrophages, the plateletsecreted exosomes targeted neutrophils would release its own DNA to form NETs (Neutrophil Extracellular Traps) upon the attack, and thus resulted in the damages of the internal organs.
By treating the dengue viral infected mice with antibodies against CLEC5A and TLR2, the survival rate reached over 90%.
Dr. Hsieh confidently said that this study found not only the critical infection pathway of dengue virus, but also unveiled a previously unknown function of EVs. “It would be a whole new chapter once we move to that direction (EVs). Since every type of cells secret their own EVs, exploring the regulatory roles of EVs in diseases other than infection may lead to powerful solutions for many other health problems. The existing research capacity in GRC, extending from Mass spectrometry, glycoproteins, and structural analysis, etc., will allow us to conduct such exciting research”, commented by Dr. Hsieh.
The Nature Communications article can be read online at: https://www.nature.com/articles/s41467-019-10360-4

