Team members(from left to right): Dr. Kuo-I Lin, Dr. Yueh-Hsiang Yu, PhD student Yung-Chieh Tseng , Dr. Alex Ma.Throughout the year WHO Centers for Reference and Research on Influenza analyze virus isolates from patients around the world and made recommendation of which circulating influenza strains will be appropriate for seasonal vaccines. Still, with all these preventive measurements, influenza epidemics continue to be a threat to the public health.
Team members(from left to right): Dr. Kuo-I Lin, Dr. Yueh-Hsiang Yu, PhD student Yung-Chieh Tseng , Dr. Alex Ma.Throughout the year WHO Centers for Reference and Research on Influenza analyze virus isolates from patients around the world and made recommendation of which circulating influenza strains will be appropriate for seasonal vaccines. Still, with all these preventive measurements, influenza epidemics continue to be a threat to the public health.
The main reason is due to the fact that the make of the right vaccine can never catch up the speed of viral mutation. Currently, of type A and B influenza that mostly pass around in human, each type has several subtypes, and strains of each subtype could vary by time and region. A vaccine made according to certain strains, just may not be protective for other strains. Therefore, as the influenza virus mutate, vaccines made from its predecessor out of calculated guess just sometimes don’t seem to be the best answer.
There are many scientists looking into solving this problem, and the idea to make a universal vaccine seem far reaching but is very attractive.
In this issue of Proc. Natl. Acad. Sci. USA (PNAS), a team led by President Chi-Huey Wong, Dr. Alex Ma, and Dr. Kuo-I Lin has reported another promising step in finding the best answer to make the universal influenza vaccine. They have not only demonstrated how an engineered glycoprotein based vaccine could yield much stronger protection against various H1N1 viral strains, but also explained in detailed how in immune system it works.
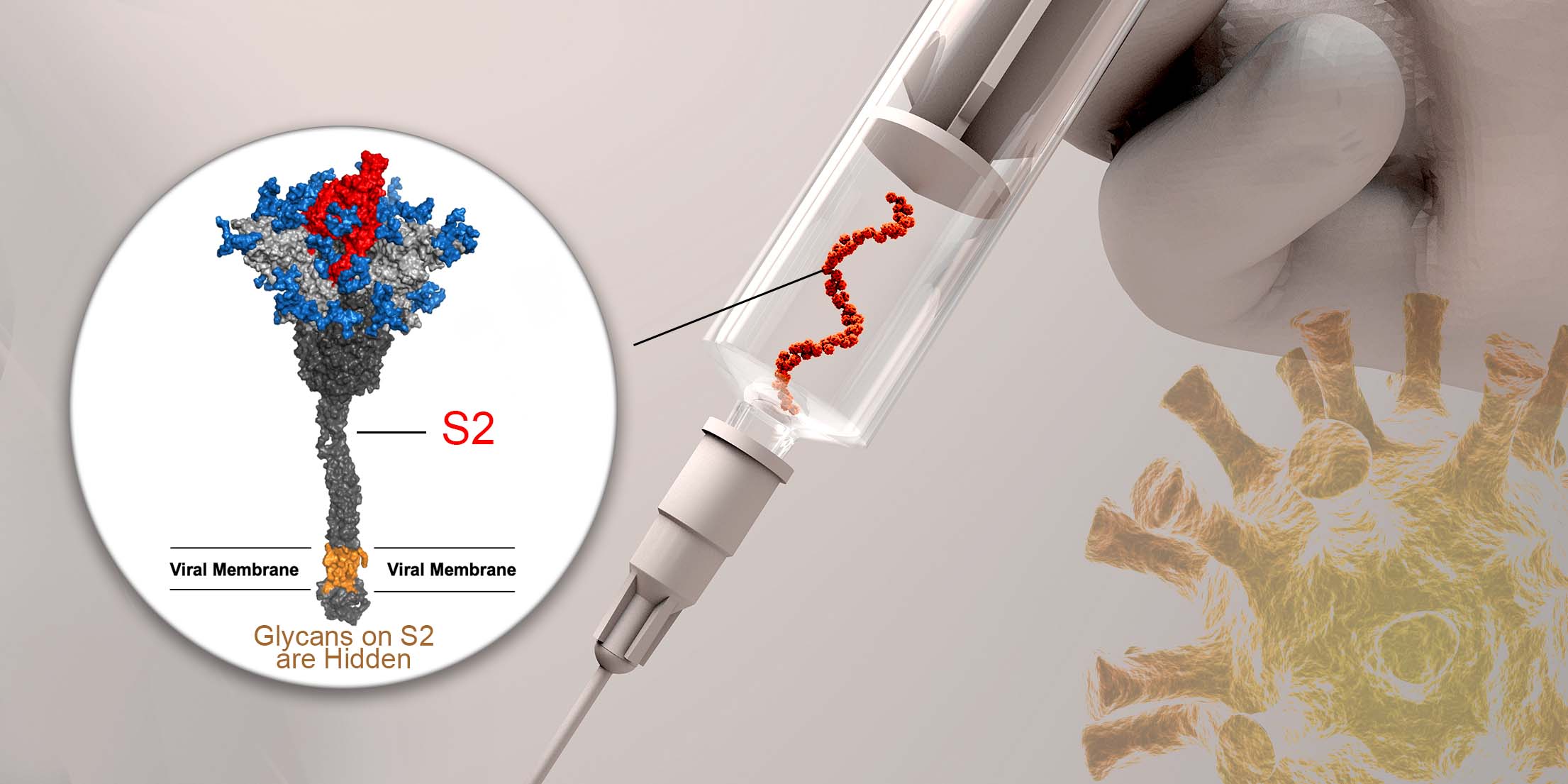
This new type of influenza vaccine is made out of an engineered glycoprotein, named monoglycosylated hemagglutinin (HAmg). HAmg is a variant of the major viral surface glycoprotein hemagglutinin on which each of the N-glycans has been trimmed down to only one sugar – N-acetylglucosamine.
Based on previous studies, the team brought up a hypothesis that the N-glycans which cover more than half the surface of HA protein act as shields to evade recognition by the immune system, such decoys significantly diversify the immune response. By removing the structurally non-essential N-glycans to expose the highly conserved protein sequences, the vaccinated monoglycosylated HA has a more “uniform presentation” of epitopes to induce better immune responses.
In the lately published article in PNAS, it described how the HAmg vaccine made from the A/Brisbane/59/2007 strain or A/California/07/2009 strain isolates can broaden the protection against various H1N1 influenza virus infections that circulated between year 1933 to 2009 in both mice and ferrets.
A number of experiments were also carried out to clarify the mechanism behind the broad protection elicited by this vaccine. They want to know, how the immune system react to it, how antibodies are induced.
Firstly, they observed better dendritic cell uptake and maturation when they incubated HAmg with dendritic cells in vitro.
Next, it showed HAmg vaccination induced better CD8+ T-cell responses. They also found that the structural integrity of HAmg is critical for better B-cell recognition and more IgG-producing cells in spleens. Thus, more antibodies are made.
After analyzing hundreds of single B-cell clones from immunized mice, they also found that HAmg vaccination induced maturation of more diverse germinal B-cell clones that can recognize a broad spectrum of HA.
Taken together, monoglycosylated HA with an intact structure and exposed conserved sequences was found to be a superior vaccine that can provide cross-strain protection against various H1N1 viruses. This may lead to a better influenza vaccine design that does not require frequent updates and annual immunizations. One major step closer to the ultimate goal of making the universal flu vaccine!
The team not only expresses high hopes in mapping out a new direction for development of universal flu vaccines, they are interested in applying the same design strategy for vaccines of other human health related virus such as Dengue, HCV and HIV as well.
The research paper can be read online at: http://www.pnas.org/content/early/2014/01/23/1323954111.abstract