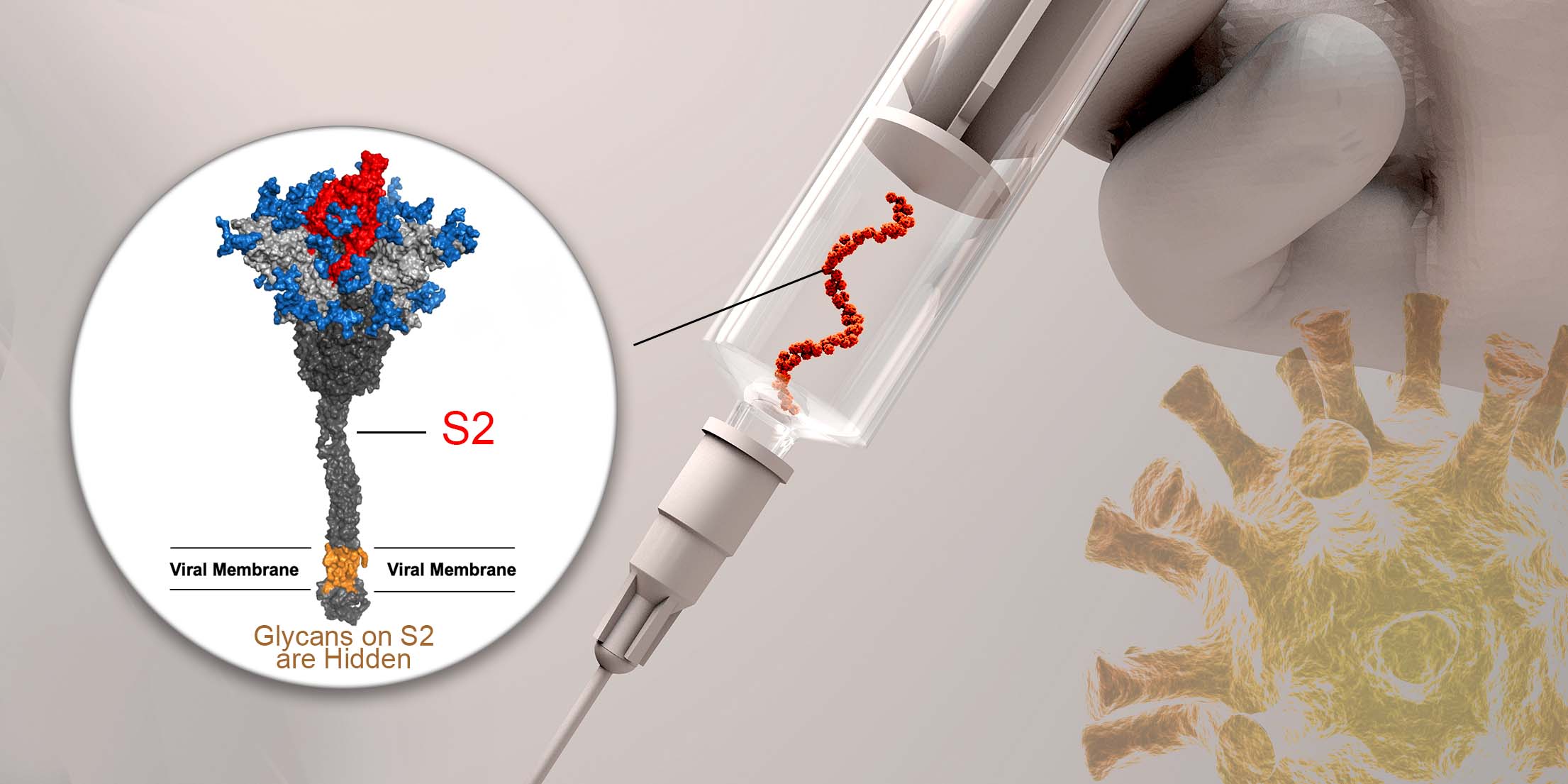
After grappling with the corona virus, SARS-CoV-2, for more than a year, the world is still being haunted by Covid-19. Remdesivir is the first drug approved by the US Food and Drug Administration (FDA) to treat Covid-19. In May 2020, Taiwanese authority also approved the use of remdesivir in patients with severe COVID-19 symptoms.
Nonetheless, Nipah virus is emerging silently and becoming an infectious disease that cannot be ignored. According to the Access to Medicine Foundation, Nipah virus had already caused a few known outbreaks in Asia. With a fatality rate up to 75%, it has the potential to become the next pandemic. Taiwanese government has issued the Nipah virus alert in early 2021.
A study published in Science Translational Medicine back in 2019 reported the efficacy of Remdesivir against Bangladesh genotype Nipah virus in African green monkeys. According to the study, all Remdesivir-treated animals survived the lethal challenge, indicating Remdesivir a potential antiviral drug for Nipah virus infections.
The synthesis of Remdesivir has been developed by Gilead Sciences. In a press release on 29th June, 2020, Gilead Sciences claimed that the entire procedure is complicated and the price of the drug can only be set as a minimum $2,340 US dollars for a 5-day course per person in clinic (https://stories.gilead.com/articles/an-open-letter-from-daniel-oday-june-29). In reality, that price tag is still a price too much to pay for many people. To ensure Remdesivir is available and affordable in desperate situations, a better drug development procedure to cut cost is inevitable.
A GRC team led by Professor Shang-Cheng Hung and Academician Chi-Huey Wong tackled this problem and successfully used a one-pot catalytic asymmetric phosphoramidation method to improve the last 2-step preparation of Remdesivir. A 10-g scale synthesis was performed with their method and afforded Remdesivir in 70% yield with a purity of 99.3%.
The methodology combined asymmetric catalysis and acid deprotection in a one-pot strategy. Regarding the final two steps of synthesis done by Gilead, Dr. Hung proposed an innovative alternative process which not only simplified procedure of purification, but also decreased solvent and silica use. On the whole, their approach both significantly improves the yield of Remdesivir and achieves a cost reduction. The study was published in the well-known international journal "The Journal of Organic Chemistry".
In the Gilead’s method, the phosphoramidoyl reagent needs to be prepared by reaction of 2-ethylbutyl-L-alanine with PO(OPh)Cl2 followed by addition of p-nitrophenol to yield a 1:1 mixture of diastereomers. Multiple crystallizations of the mixture can come up with a total 39% yield in purified (S)-P-form intermediate. That is to say, there is a significant waste of 61% of the chemicals used. In addition, coupling of the (S)-P-phosphoramidoyl reagent with the primary alcohol furnished the (S)-P-phosphoramidate (70%), which was hydrolyzed under acidic conditions to give Remdesivir in 69% yield. Purifications of the products by silica gel column chromatography are needed in both steps.
To solve this problem, the GRC team employed a chiral bicyclic (S)-carbamate derived from imidazole and cinnamaldehyde as a catalyst. The chiral property of the catalyst makes the selectivity in favor of the desired (S)‐P-form intermediate. Futhermore, the unfavorable steric hindrance in the (R)-P-form intermediate could equilibrate to the (S)‐P-form intermediate for further coupling with the primary alcohol. As a result, 96.1% diastereselectivity of the (S)-P-phosphoramidate could be achieved.
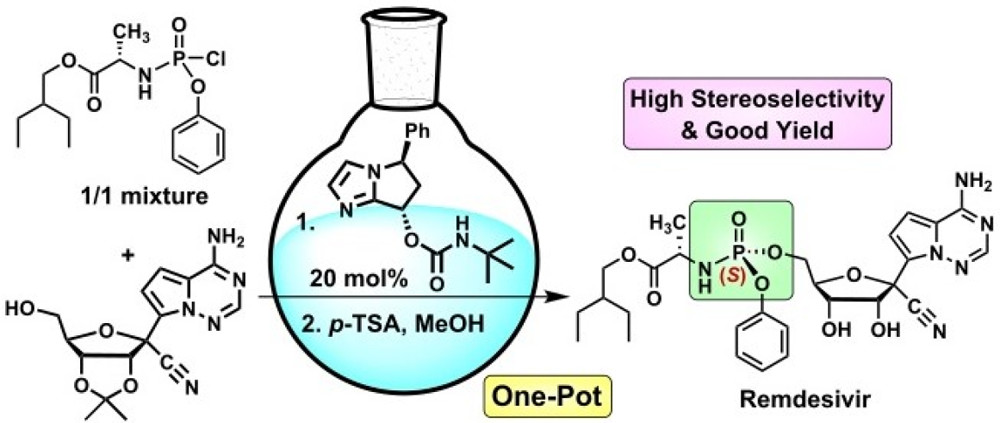
Next, with a one-pot approach, they managed to create a process carried out in the same reaction flask via a combination of (S)-P-phosphoramidation and acidic hydrolysis, and eventually provide a mixture of raw product, which was further purified via recrystallization to make Remdesivir.
The one-pot strategy increase the overall yield for the last two steps from 48.3% to 70%, plus, the organo-catalyst can be recovered for reuse.
All in all, they have delivered a proven method and filed a patent for it. The technology is potentially able to be transferred to an API company for mass production in the future.
Should COVID-19 morphs and lingers, or Nipah if joins the pandemic hassle and create a double whammy effect, by law, Taiwan can request Gilead’s authorization to manufacture the drug locally. This research has provided a new technology for making Remdesivir much more effectively and economically.
The paper titled “Practical Remdesivir Synthesis through One-Pot Organo-Catalyzed Asymmetric (S)-P-Phosphoramidation” can be read online at: https://pubs.acs.org/doi/10.1021/acs.joc.0c02888.

Selected Cover Photo can be found online at: https://pubs.acs.org/pb-assets/images/_journalCovers/joceah/joceah_v086i007-2.jpg?0.8371290252827563
(The image of SARS-CoV-2 is presented as origami art. The top-left and bottom-right photos are based on the structures of C60 and C80, respectively. Each “carbon” unit in green was folded from a regular triangular paper. 60 and 80 “carbon” units were assembled in different compositions to form C60 and C80, respectively. The red unit represents the spike glycoprotein trimer. It was also folded from a regular triangular paper with three feet to stand on the “carbon” unit.)