Yearly, as the temperature goes down, it is inevitable to hear about flu shots, for it has already become a seasonal event for the sake of getting protections from the flu.
Yearly, as the temperature goes down, it is inevitable to hear about flu shots, for it has already become a seasonal event for the sake of getting protections from the flu.
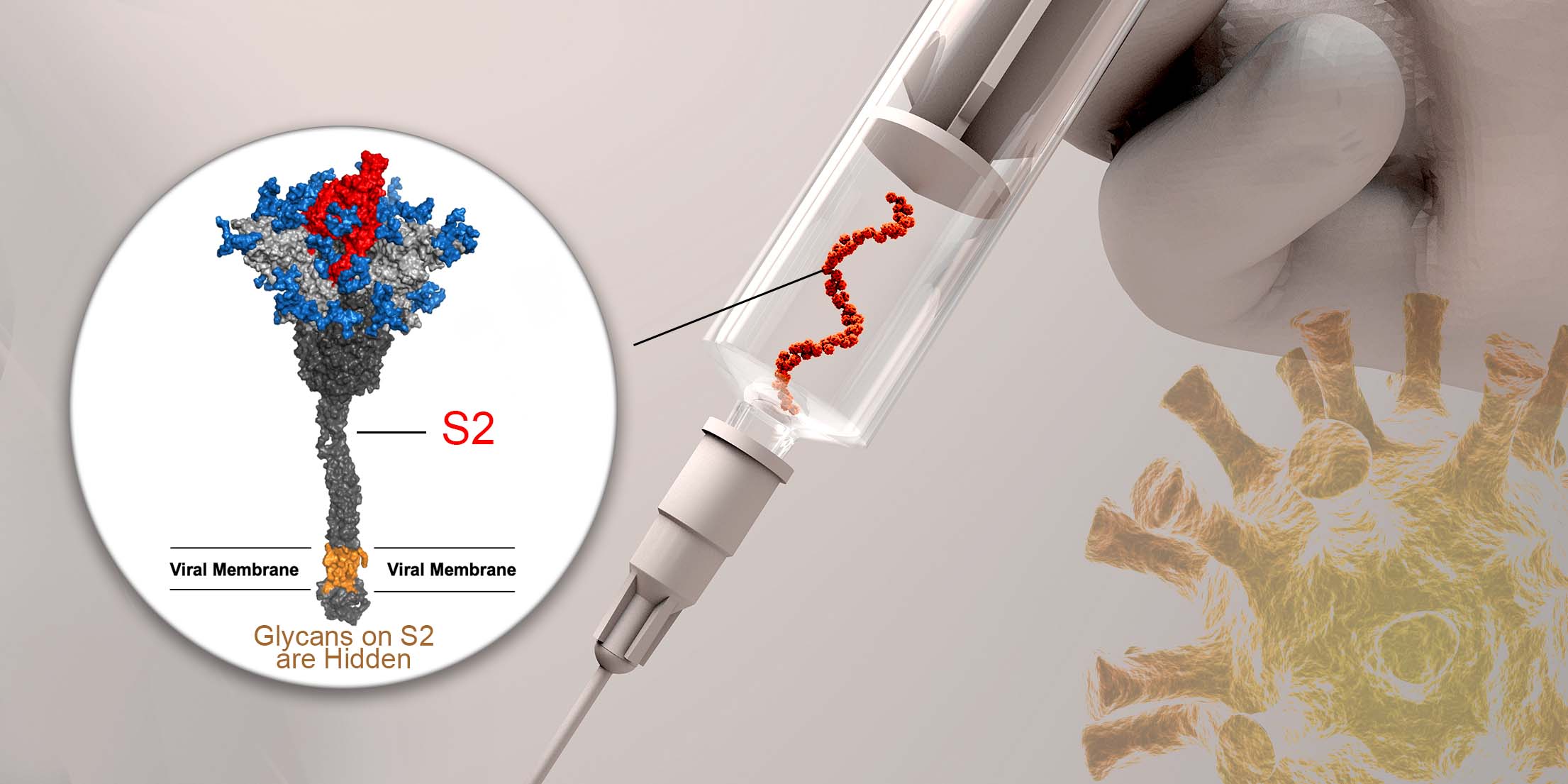
As indicated in the U. S. Centers for Diseases Control and Prevention website, currently the global health organizations (130 national influenza centers in 101 countries) work closely to conduct year-round surveillance and predict trends of influenza activity for the purpose of manufacturing preventive flu vaccines which target against the selected viral strains. However, vaccine production time takes up to half of year, and there is still a great chance that the vaccine does not prevent influenza disease if the circulating strains do not match well with the prediction, or when outbreak of new virus strain takes place, as seen in 2009 swine flu pandemic. It is therefore important for future flu vaccine design to provide broader protection against variety of virus strains and to shorten manufacturing time.
 In the September print issue of the journal Trends in Biotechnology, President Chi-Huey Wong and Genomics Research Center Investigator Che Alex Ma published a review article along with a vivid cover page diagram to discuss the details of the influenza virus, historical pandemics, and current and future vaccine design.
In the September print issue of the journal Trends in Biotechnology, President Chi-Huey Wong and Genomics Research Center Investigator Che Alex Ma published a review article along with a vivid cover page diagram to discuss the details of the influenza virus, historical pandemics, and current and future vaccine design.
The highlight in this article, “Understanding how glycans on HAs affect the immune response and knowing how broadly neutralizing antibodies are induced for immunity”, will pave the way for a cross-protective influenza vaccine that does not require frequent updates or annual immunizations.
The full article can be read online on Trends in Biotechnology website.