In the battle against cancers, more and more attention are shifted toward the surrounding environment, not just of the environment of the patients, but also the surrounding matters of the cancer cells within the patients.
When tapping into the microenvironmental study about cancers, scientists are looking into all kinds of elements identified around the cancer cells, it can be fibroblast cells, immune cells, or fat cells; it can also be a chunk of tissues or tiny molecules.
In the microenvironmental study of breast cancers, Dr. Wen-Hwa Lee’s team has identified lately the scenario for how breast cells thrive in a neighborhood full of adipose tissue. Adipose is a biological term for the body fat that we know of.

Team members(from left to right):Dr.Wen-Hwa Lee, Dr. Chun-Kai Huang and Dr. Chun-Mei Hu.
Many researchers had already proved close relationships in between body fat and chronic diseases, and cancers. As for breast cancer, clinical observations indicated higher risks for women with larger breasts as well. Furthermore, poor prognosis seemed to happen more to clinical cases when breast cancers had already invaded into the fatty tissues. All these clues encouraged Lee’s team to look into it.
In the Journal Nature Communications published on March 10th, First author Dr. Chun-Kai Huang detailed how they traced all the way to epigenetic modifications and proved the role of fatty tissues surrounding breast cancer cells. Ultimately, according to their finding, the fatty cells are responsible for providing a nourishing environment to trigger the breast cancer cells multiply.
Unlike known studies that tackle this issue from the perspective of cancer cells, Lee’s team decided to study from the fatty tissue biopsy. By collaborating with Dr. King-Jen Chang and Dr. Wen-Hung Kuo of National Taiwan University Hospital, the team obtained adipose tissues derived from breast cancer operating rooms. Through a method called co-culture, they grew layers of fatty tissues with various breast cancer cell lines.
With Micro Array analyses and RNAi knockdown screenings, from a number of 16 membrane related genes, they identified a gene called monocarboxylate transporter 2, also known as MCT2, that plays a crucial role in this cancer/fat culture model.
Existing knowledge about MCT2 is that it is in charge of trafficking. Prior research has described MCT2 as a transporter on the cancer cell membranes allowing in-and-outs of three specific molecules, namely, Pyruvic acid, Lactate, and beta-Hydroxybutyric acid (β-HB), which are also can all be found in fatty tissues.
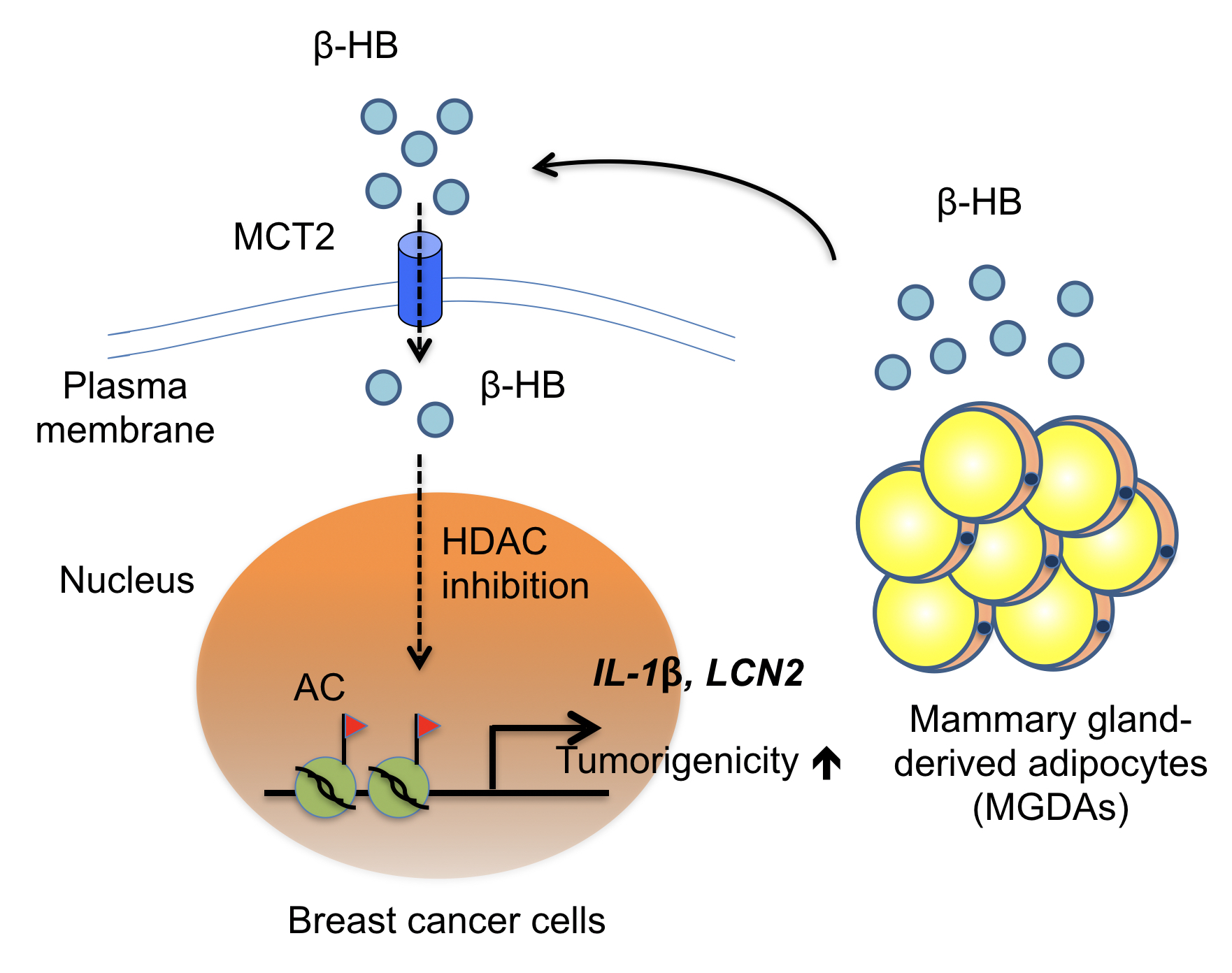
Heterotypic interaction between MGDAs and MCT2-expressing breast cancer cells in the breast tissue microenvironment
As they drilled deep into this microenvironmental activity, further experiments showed that β-HB contributes to MCT2 expressing breast cancer progression. β-HB functions as an endogenous HDAC inhibitor to increase the expressions of several tumor-promoting genes, including two growth factor genes IL-1β and LCN2 in breast cancer cells.
In conclusion, in the neighborhood of breast cancers when lots of fatty tissues can be found, if the breast cancer cells also have MCT2 on the membrane, then, MCT2 will keep the supply of β-HB and nourish the breast cancers. Therefore, MCT2 can be a target to find therapeutics down the road!
The article of this research can be found at the website of Nature Communications at:http://www.nature.com/articles/ncomms14706


