Since the first case of AIDS was diagnosed in 1981, more than 35 million people have died from the HIV/AIDS. Based on the statistics published by the Joint United Nations Programme on HIV/AIDS (UNAIDS), there were 36.7 million HIV carriers in 2016. Right now, around 2.5 million people die of AIDS each year, and there still lack treatments to effectively cure this disease. Scientists believe that only a successful HIV vaccine can eradicate the virus; however, more than ten HIV vaccines have been tested but failed in the clinical trial phases.
Recently, scientists isolated a group of broadly neutralizing HIV-1 antibodies (bNAbs) from patients infected with HIV, the finding raised a new hope for HIV vaccine development because the ligands of bNAbs could be used for the design of effective HIV vaccine.
In the last few years, studies showed that bNAbs bind to specific glycans on gp120, a glycoprotein expressed on the surface of HIV envelope. The gp120 is a heavy glycosylated glycoprotein and the weight of its glycans is about half of its molecular weight. Moreover, the glycans on gp120 have highly complicated structures. Furthermore, many bNAbs require the synergy of binding to two different glycan molecules to effectively interact with the virus, complicating the analysis of bNAbs binding glycans.
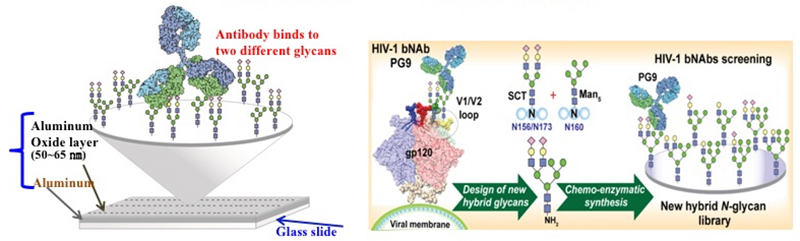
Two years ago, the research team led by Dr. Chi-Huey Wong and Dr. Chung-Yi Wu of Genomic Research Center successfully constructed a modular synthetic strategy to rapidly synthesize N-glycans on gp120. Along with the newly developed aluminum oxide coated glass (ACG) glycan array platform developed by the same team, the glycans recognized by bNAbs can be rapidly screened.
Previously, the research team identified the synergy of two N-glycans to promote binding to bNAbs. In the newest study, they synthesized a library of hybrid N-glycans by the previously developed modular method and created an ACG array to identify a hybrid glycan to mimic the binding of these two N-glycans.
|
| Figure 1: (a) With the high density and high homogeneity of the ACG slide, two different sugar molecules can be printed on the same spot to quickly screen the glycans that can synergistically bind bNAbs. (b) By using the previously developed modular N-glycan synthetic strategy, two selected glycans from ACG slide can be assembled into one hybrid type N-glycan. |
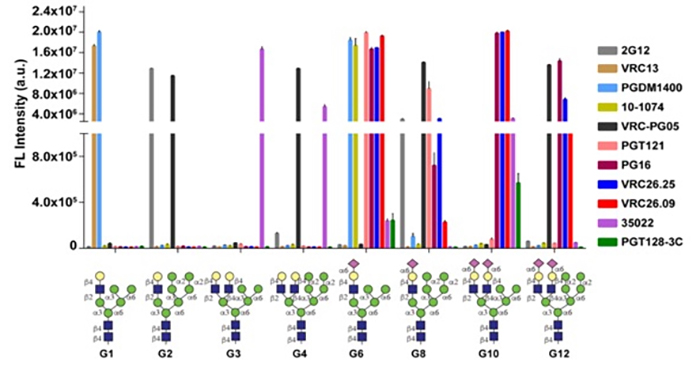
Then, the research team used more than ten effective bNAbs provided by Professor Dennis R. Burton of Scripps Research Institute and Professor Peter D. Kwong of the US National Institute of Health, and conducted further hybrid N-glycan binding analysis on the AGC array. The results showed that hybrid glycans G6 and G10 strongly bound to most bNAbs. In particular, the hybrid glycan G10 bound better with the bNAb PG9, than the combination of two glycans.
|
| Figure 2: Binding affinity of various hybrid N-glycans with different bNAbs. G6 and G10 have strong binding affinity to various bNAbs. |
The team proposed that these unusual hybrid N-glycans probably are formed when gp120 was synthesized by HIV, and thus trigger the immune response to produce antibodies against the unusual glycan structure. Hence, these hybrid glycans could be used as antigen for the design of carbohydrate-based HIV vaccines.
This work has been recently published in Journal of the American Chemical Society. According to the first authors Dr. Vidya S. Shivatare and Dr. Sachin S. Shivarare, the research teams are currently using G6 and G10 glycans to develop the HIV vaccines.
The complete research article can be read online at: https://pubs.acs.org/doi/pdf/10.1021/jacs.8b00896


