“Bad Knees” is not an unfamiliar topic among older folks. Most of the complaints are pains and swellings, accompanying clicking and locking at the joints. Some people believe it’s a sign of ageing when one starts feeling funny at the knees. And it may just be true!
Of the many knee complications, osteoarthritis (OA) is the most common chronic joint disease. It is a condition believed to be linked to age, obesity, joint deformity, injuries, and even gender. OA can happen not just to knees, other joints like the hip joints and knuckles can experience the symptoms as well. Somehow, OA favors older female more than other age/gender groups.
Although the disease has been long recognized, the exact cause remains unknown. A team led by Professor Shang-Cheng Hung, Professor Chi-Huey Wong and Dr. Rachel Cheng dug into this problem around seven years ago. Now, a report of how a trisaccharide structure was proved to be the ultimate substrate to en-do-O-sulfatase-1(Sulf-1) has been published. They have not only unraveled the key to a known mystery but also proposed a structure as a potential inhibitor to solve the problem. The detail was published in Journal of the American Chemical Society (JACS).
The study was initiated after they attended an OA discussion with doctors. Sulf-1 was reported to be found abundant in the tissue fluid in knee areas from OA patients. It is believed that at the beginning of the onset, proteoglycans on the surface of cartilage had some kind of molecular shifts, where the sulfate of the glycosaminoglycan (GAG) was first removed by sulfatases, a type of enzyme, and after that, a series of removals of the glycans and proteins are carried out, eventually led to broken cartilage.
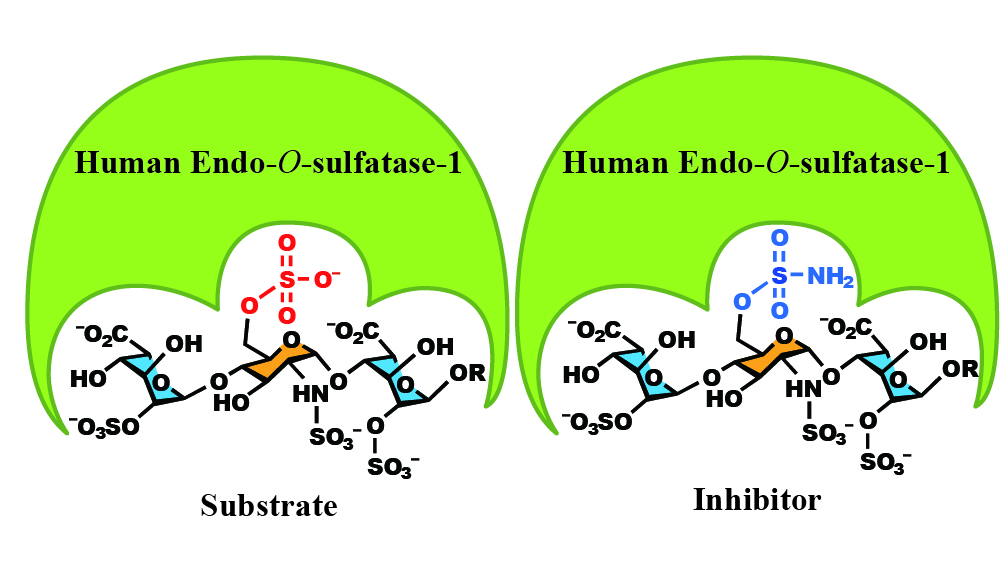 |
| Figure: Identified trisaccharide(left) and inhibitor(right) |
There are totally 17 types of sulfatase in human, including Sulf-1. Gly-cosaminoglycan (GAG) is a complex glyco-molecule with multiple sulfate groups attached to various locations. The team was obsessed by its com-plexity and unknowns. They’d like to find out how Sulf-1 breaks apart the whole structure. If they can find the key, a solution to the problem can then be realized.
By working with hospital physicians, they retrieved the original specimen and extracted the Sulf-1 enzyme, started the study together.
The chemistry attraction to Sulf-1 is heparan sulfate proteoglycan (HSPG) in glycosa-minoglycans (GAGs). GAGs are long linear polysaccharides biosynthesized by repeating disaccharide units.
The team decided to take a systematic approach, they started from making disaccharide one after the other. After making three types of HSPG varied by length: disaccharide (2 sugar units), tetrasaccharide (4 sugar units), and hexasaccharide (6 sugar units), they could test to see which one could be a candidate as a substrate to Sulf-1.
Ms. Li-Ting Chiu, the first author of the publication, came up with a competition-based assay. Chiu explained that in order to find the best candidate, she added a fluorescent substance 4-MUS to the mix. 4-MUS is a commercially available substrate that can also bind to Sulf-1, although not a good substrate, but it will have a glowing effect after release of the sulfate anion to 4-MU. By including 4-MUS, Chiu could tell if any of the can-didates can outperform 4-MUS, if so, it will not glow. By this method, only the tetra-saccharide and hexasaccharide proved to have strong interaction with Sulf-1.
With that clue in mind, they did a calculated analysis, and a total of 16 tetrasaccharide compounds with different sulfation patterns were selected from the existing library to further find a best candidate.
Finally, the mystery was solved! The 6-O position of the D-glucosamine was identified as an active site for the whole process. That is a hotspot where Sulf-1 removes the sulfate group from the HSPG and triggered the whole damaging processes down the road.
The key to design an ultimate drug candidate is to make it as simple as possible, therefore, they did further analysis and further trimmed the 4 sugar compound to a trisaccharide, and proved it to be the most minimized element that is the key substrate element to the Sulf-1 enzyme.
To create an inhibitor, they used the trisaccharide as the base, and by replacing the sulfate group at the 6-O position by a sulfonamide group, it proved to have a stronger binding affinity to Sulf-1, and thus, a potential inhibitor was created!
With this chemical model that explains the cause-effect of OA at the molecular level, the team not only achieved another milestone in glyco-synthesis, but also has provided a solid ground work for the next level, namely the cell and animal studies. They have high hopes with using Sulf-1 as a treating OA early onset target, and this trisaccharide opens up a door to eventually ease pains for OA suffers.
The original article can be read online at the JACS website: https://pubs.acs.org/doi/10.1021/jacs.0c00005


