
Figure 1: From left, Yu-Peng Hu, Professor Shang-Cheng Hung, Shu-Yi Lin

This research was also reported in the 06/27/2011 issue of C&EN.
When it comes to solving the living problems, biologists and chemists usually team up to analyze and recreate. After all, all nature’s creations can be broken down into molecules and chemical elements.
While the breaking up and analysis is difficult, finding a solution and making it work is beyond ordinary imagination. That is why when a specific octasaccharide can be created in lab, and proven to block viral entry into the host cells, and could be considered as a feasible preventive drug lead, has made all excited.
| Herpes simplex type 1 virus & Heparan sulfate |
In the June online issue of Nature Chemistry, a team in Academia Sinica has announced their work of two successfully synthesized cell-surface octasaccharides, both compounds have proved effectively inhibiting the infection of herpes simplex type 1 virus, commonly known as cold sore virus.
Dr. Shang-Cheng Hung, a research fellow in the Genomics Research Center, has dedicated decades of efforts in studying and synthesizing glycans, or, in simple terms, sugars!
Dr. Wen Chang, a research fellow from the Institute of Molecular Biology, is a virologist who focuses on vaccinia virus. Her interest in knowing how the virus enters into host cells has led her to investigate heparan sulfate (HS), which is a type of glycan.
Hung and Chang started the collaboration way back in 1998.
Heparan sulfate (HS) is a linear polysulfated polysaccharide that is found abundant on the cell surface. It is known as a key point for many kinds of virus, like pox, dengue, hepatitis C, and herpes (including chicken-pox), to get into a living cell by binding itself to HS. However, since HS is really a clustering of many kinds of glycans with different sulfation patterns, unless there is a way to separate all these glycans into single elements, the exact interactions will remain as mysteries. The trouble is, HS seems to be delivered in a packaged deal by nature.
A study published in 2002 has suggested a 3-O-sulfonated octasaccharide that’s found on HS can bind to herpes simplex type-1 virus. So, Hung and Chang rolled up their sleeves to begin a project to use the synthesized octasaccharide to study herpes type-1’s infection.
An octasaccharide can be thought of as a glycan with 8 monosaccharide residues.
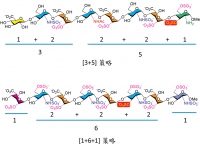
Figure 2: successfully made the compounds by [1+6+1] and [3+5]
Glycan is such a substance that’s mysterious and fascinating. All glycans are constructed from a C•(H2O)n (where n ≧3) form. A hydroxy group, that is, an Oxygen and a Hydrogen (–O–H) connected hand-in-hand, is where a glycan interacts with others to transform into a different substance. Moreover, its 3D structure makes the position of a hydroxy group significant, because that can determine the characteristics of such a chemical compound.
In order to synthesize a complicated glycan, one needs to come up with sophisticated strategies to design steps for connection of a certain –OH group to an added substance, and meanwhile using ways to shield other –OH groups from being exposed and possible interactions. The steps may be lengthy and requires extensive chemical knowledge.
| How to make [1+6+1] and [3+5] octasaccharides |
Hung has chosen a journey with pretty much no precedent, because he deeply believes if he can find a way to mimic complex glycans, the way to resolve infection problems will be much easier. “It could have been the childhood impression from experiencing the Dengue fever fright that drove me to keep going.” commented Hung.
Indeed it’s a thorny journey, started in 2002, his team had finished up to connecting five saccharide units and then stumbled. Until 2007, when he came up with a highly regioselective one-pot protection strategy for carbohydrates, reported in the top-fly Nature journal, to foster a much simplified production mechanism for glycans, he’s already accumulated from trial and error experiences more than anyone could imagine nor compete.

Figure 3: Dr. Wen Chang
In this newly published research, they have gone through 47 and 57 steps in total to make two octasaccharides, respectively. The whole experience is sort like solving a diabolical puzzle, just when they thought it’s close to completion, they would have recognized they’ve reached a dead end, and everything will have to be reset and started all over again. Finally, two of the graduate students each successfully made the compounds by [1+6+1] and [3+5] approaches separately.
The final validation executed by Dr. Chang’s lab has shown both compounds have inhibited approximately 50% of the infection (IC50) at 5.4 and 3.9 microgram per ml concentrations by using the cell line of monkey.
“As opposed to eliminating the virus after it’s already inside the host, if we can come up with ways to stop the virus at the front gate, it provides a new direction of antiviral development.” commented Chang. Dr. Chang has a high expectation on the Hung’s team to eventually produce a whole library of such compounds to resolve similar problems created from other viral types.
The original paper “Synthesis of 3-O-sulfonated heparan sulfate octasaccharides that inhibit the herpes simplex virus type 1 host-cell interaction” now can be read online.


